بِسۡمِ اللهِ الرَّحۡمٰنِ الرَّحِيۡمِ
Summary of key points re Fluenz® vaccine:
The Fluenz® nasal flu vaccine is formulated to offer preventive protection against influenza among children and adolescents aged 24 months to less than 18 years. Administered as a nasal spray, it is provided free of charge to eligible children by the National Health Service (NHS) in the U.K. on an annual basis, either at general practices or schools. In addition to safeguarding the child against influenza, this nasal spray vaccine contributes to reducing the transmission of the infection from the vaccinated individual to their family members, caregivers, and the broader community.
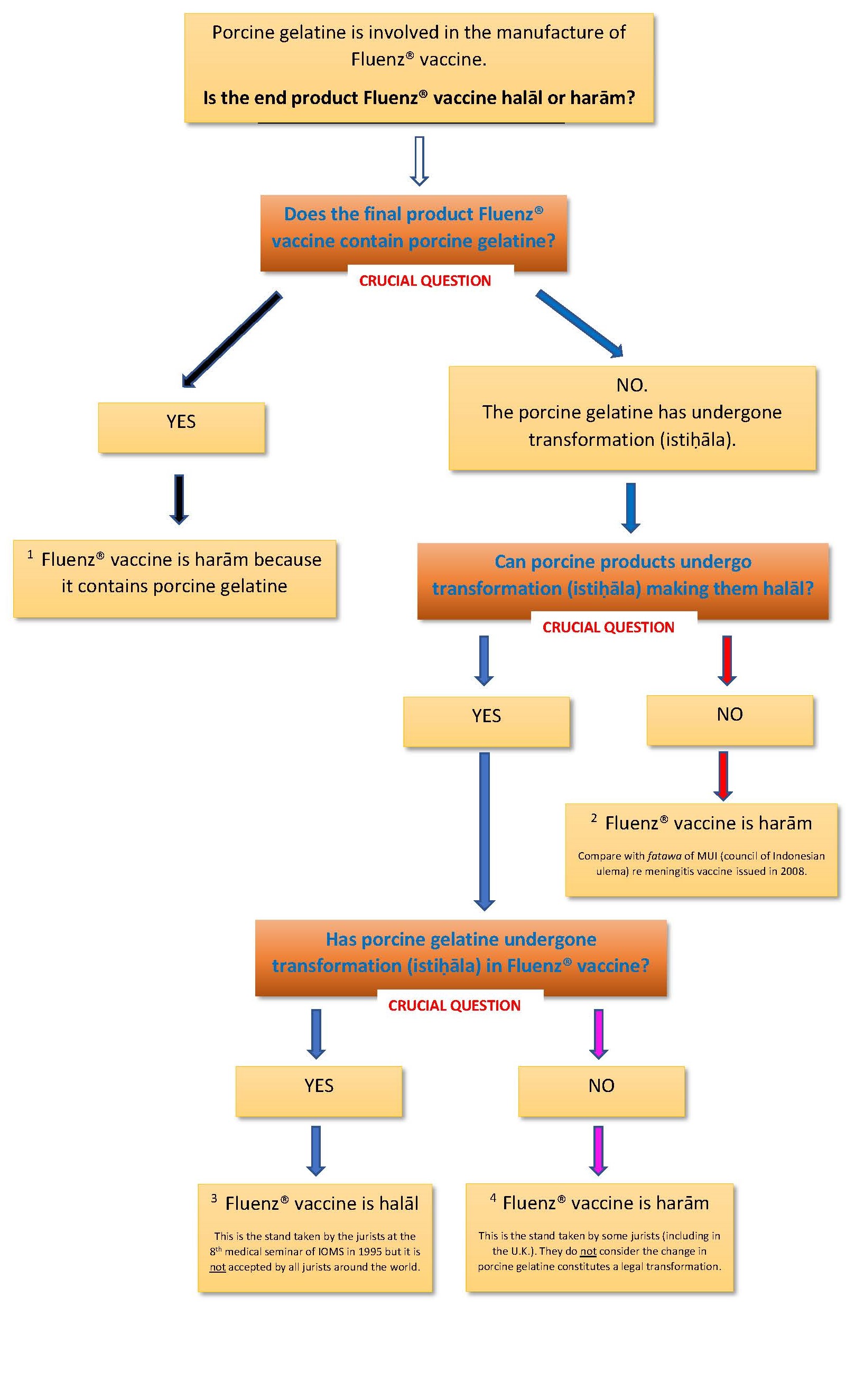
The Fluenz® vaccine contains porcine gelatine. See letter from the manufacturer AstraZeneca at bottom of this page.
- Porcine gelatine serves as a stabilizer for the live attenuated virus in the Fluenz® vaccine during its manufacturing process.
- Porcine gelatine is purified so it is identical to cow gelatine, goat gelatine or other animal gelatine. Some scholars consider this to be an important factor in their ruling on the issue.
- Despite porcine gelatine being used, it undergoes extensive degradation into peptides, rendering it undetectable through sensitive scientific tests for pig DNA in the Fluenz® flu vaccine. Consequently, the source of the porcine gelatine cannot be discerned.
- Certain Muslim jurists, such as those adhering to the Shafii school of thought, do not accept the principle of istiḥāla (transformation), which allows a judicially impure substance to become pure. Consequently, they deem the Fluenz® vaccine containing porcine gelatine as harām, irrespective of the extent of its transformation.
- In 1995, at the 8th medical seminar organized by the Islamic Organization for Medical Sciences (IOMS) in Kuwait, over 100 prominent Muslim jurists and experts issued a fatwa asserting that porcine gelatine in vaccines is judicially pure and permissible for use based on the principle of istiḥāla. This fatwa designates the Fluenz® vaccine as halāl, although it is not universally accepted among all Muslim jurists.
- Around 2014-2015, some Muslim jurists, particularly those of the Hanafi school in the UK and elsewhere, do not recognize the changes undergone by porcine gelatine during the manufacturing process of the Fluenz® vaccine as constituting istiḥāla, thus maintaining their stance that the vaccine is harām. See below fatwa endorsed by Muslim Council of Britain below.
- In September 2020, the British Fatwa Council, possibly affiliated with the Karimia Institute in Nottingham, issued a fatwa affirming the permissibility of the nasal flu vaccine containing porcine gelatine. This fatwa, signed by Mufti Dr. Hafiz M. Munir Al-Azhari, Mufti Yar Muhammad Khan Qadri, and Dr. Musharraf Hussain Al-Azari, allows for its use.
- For healthy children within the recommended age range, the Fluenz® vaccine remains the most effective option. However, an alternative injectable form of the vaccine, devoid of porcine products, is available.
- Muslim jurists who consider medicines, including vaccines, containing porcine components as harām permit their use in cases of necessity (darurah).
Dr. A. Hussain, 2017